Business Contents
Services provided by “Science Globe Corporation”

Science Globe Corporation offers four business services.
- ○ GCP Audits (Overseas and Domestic audits)
- ○ Medical Writing / SOPs Prep & Rev / Translation
- ○ Education & Training / Lectures
- ○ Strategic Consultation
GCP Audits (Overseas and Domestic audits)
The head of GCP audit has a rich experience of overseas and domestic audits. He conducted GCP audits in China, Korea, and Taiwan in 2009 and in China in 2011. Our company can conduct a GCP audit in a high quality quickly and provide clients with audit reports on their studies to meet regulatory requirements.
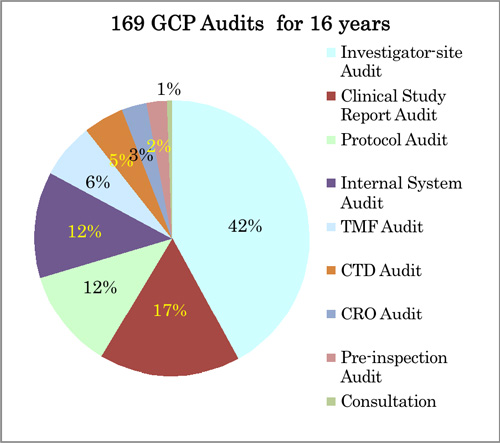
1. System Audits
1) Internal System Audit (Audits of Clinical Organization, Clinical SOP, Education, Monitoring, DM, CSV, Biostatistics, Medical Writing etc)
2) External System Audit (CRO, CSV, Laboratory etc)
2. Study-specific Audits
1) Protocol Audit
2) Investigator-site Audit
3) Clinical Study Report Audit
4) TMF Audit
5) CTD Audit
3. Support for Inspections (Pre-inspection audit, mock inspection)
- Fig. 2 GCP Audit Experiences
Medical Writing / SOPs Prep & Rev / Translation
The head of Medical Writing has strong background in medicine (PhD in Medicine), and has a 5-year working experience as Senior Medical Writer in a global environment at a German affiliated pharmaceutical company. She can make the following documents:
1. Protocol
2. Investigator’s Brochure
3. Clinical study report
4. NDA application dossier (CTD)
The person in charge of making SOPs has prepared audit SOPs for 3 pharmaceutical companies. These SOPs are concise, to-the-point, and user-friendly. He can make global l SOPs on clinical studies and audits in English and Japanese bilingually.
1. Preparation/revision of domestic clinical SOPs
2. Preparation/revision of global clinical SOPs
Our company will translate clinical regulations in various countries (in English, Korean and Chinese) and other relevant books into Japanese and publish their Japanese editions. The person in charge had published several papers in foreign science journals as a scientist and has published 3 books on Korean clinical regulations, authority organization, and inspection translated into Japanese.
Education & Training / Lectures
The trainer can teach the CRA/GCP auditor candidates domestic regulations including J-GCP in an understandable manner according to the systematic curriculum. The trainer will educate global GCP audit candidates about EU, USA and Asian regulations including ICH GCP. The trainer has many experiences in giving lectures (total 14 times) to the public including explanation to CRAs in a CRO and GCP auditors in a big pharmaceutical company, twice respectively.
1. Planning/performing introductory education/ training
2. Planning/performing continuous education/training
3. Clinical regulations and circumstances in Asian countries (Korea, China, Taiwan, Singapore etc.)
4. Topics of clinical studies and GCP audits in tripartite (EU, USA and Japan) and Asia.
Strategic Consultation
We can conduct a consultation on various duties from the beginning of a clinical study to NDA submission under the supervisory of a specialist of biostatistics. Although we do not conduct data management or biostatistics, we can introduce you some proper companies. In case that you have a plan to develop a pharmaceutical medicine or device in Asia, we can provide you with consultation services in corporation with some proper CROs in the corresponding country.


株式会社 Science Globe
〒220-0003
神奈川県横浜市西区楠町7-1-1805
(地図)
045-620-6092
045-620‐6093
・GCP監査(海外/国内)
・SOP作成/改訂
・CRA/Auditor研修
・DM/統計解析
・Medical Writing
・開発戦略コンサルティング


